
With The Innovative Brand AiMediQ PathFree Technologies Has Multiple Devices In Their Final Stages Of Development
PathFree Technologies Corporation is proud to announce the brand AiMediQ and that we have been working diligently on developing several innovative products, which are now in their final stages of development. Our team of experts has been tirelessly working on bringing these cutting-edge products to life, and we are excited to share them with the world.
At PathFree Technologies, we understand the importance of protecting intellectual property, which is why all of our inventions are patent protected. This ensures that our products are unique, and our customers can rely on us to deliver quality solutions that are unparalleled in the market.
We are committed to keeping our customers updated on the latest developments, which is why we will be announcing our new products on our website as we get closer to releasing them to the medical community in the United States and globally.
We are confident that our innovative products will revolutionize the medical industry and improve patient outcomes. Our team is dedicated to creating solutions that meet the unique needs of healthcare professionals and patients alike.
.

DataSyteAiCLS involves integrating advanced technologies, artificial intelligence, machine learning, and automation to create a smart and efficient code cart for use in medical emergencies, particularly during cardiopulmonary resuscitation (CPR) and advanced cardiac life support (ACLS) situations.
DataSyteAiCLS is a revolutionary device designed to assist medical response teams during emergency situations, particularly in the case of cardiopulmonary arrest. This is a life-threatening condition that requires immediate attention to save a patient’s life. The response team is typically composed of physicians, nurses, technicians, and other healthcare professionals who work together to provide Advanced Cardiac Life Support (ACLS) procedures.
The ACLS protocols, which are published by the American Heart Association and the International Liaison Committee on Resuscitation, provide a set of clinical guidelines for the primary treatment of life-threatening cardiovascular emergencies, such as cardiac arrest, stroke, and myocardial infarction. These guidelines are set into several groups of “algorithms,” which provide a standardized set of instructions to increase the effectiveness of the treatment.
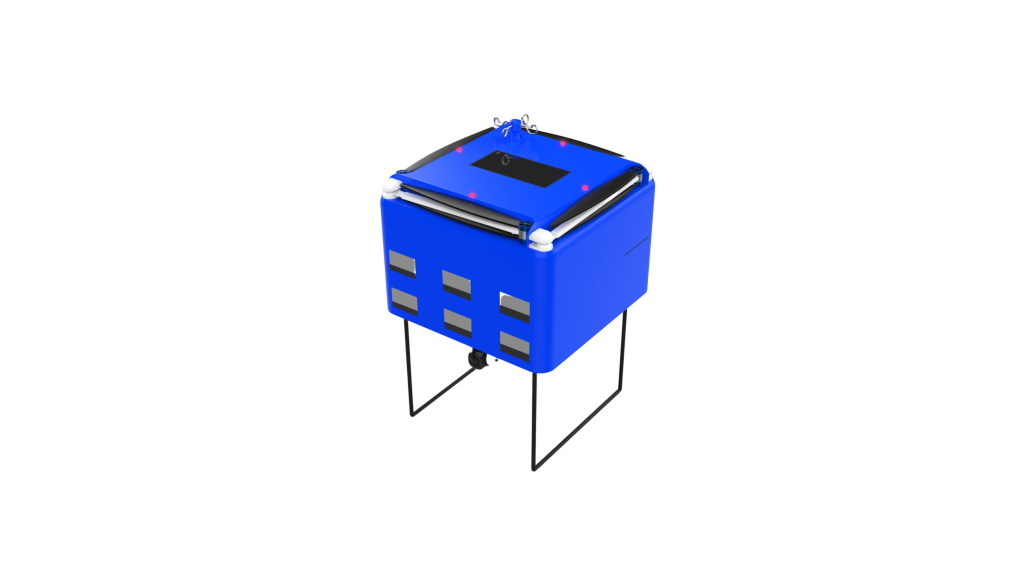
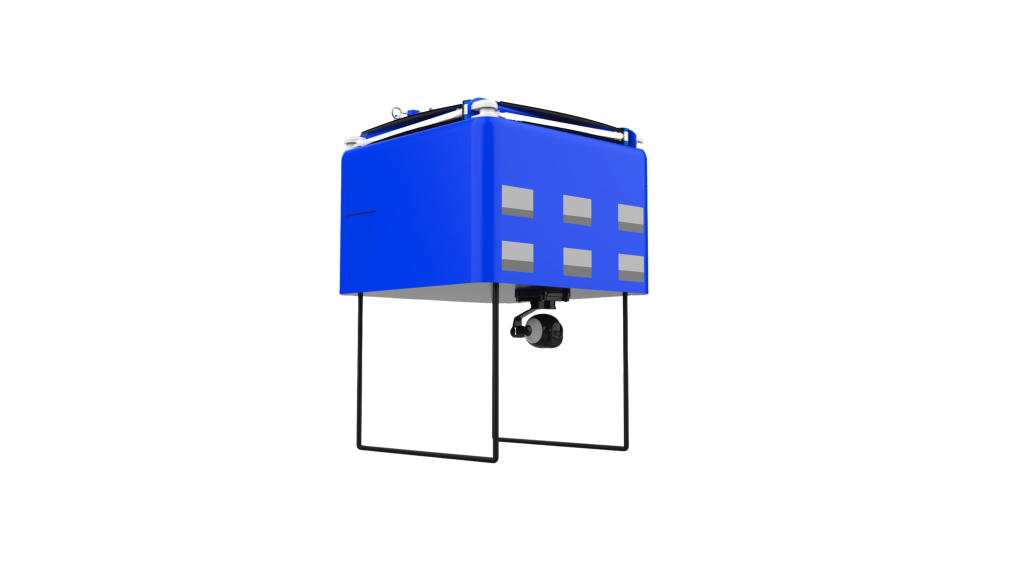
DataSyteAiCLS is designed to support the response team by reducing the knowledge gaps and potential human errors that may occur during the performance of ACLS procedures. DataSyteAiCLS incorporates a control unit, which is equipped with a processor, memory, cameras, display, input devices, and a set of drawers that contain medicines and medical equipment needed for resuscitation procedures.
DataSyteAiCLS is able to receive information about the patient’s condition and automatically identify the appropriate standard protocol for performing the cardiopulmonary resuscitation. DataSyteAiCLS can also calculate the dose of medicine based on the patient’s weight and provide step-by-step instructions to guide the response team through the proper procedures.
Furthermore, DataSyteAiCLS can analyze images captured by its cameras to recognize the actions and gestures of the response team during the resuscitation process. DataSyteAiCLS can issue alerts to the response team if any errors occur during the procedure, reducing the risk of potential complications.
Other features of DataSyteAiCLS include the ability to retrieve patient records, update patient records with intervention details, retrieve diagnostic and monitoring data from the patient monitoring system, locate medicine and equipment within DataSyteAiCLS records the procedure for later analysis and training, provide visible and audible alarms for various unsafe conditions, provide simulated training of ACLS protocols, and maintain a running inventory of medical supplies contained within DataSyteAiCLS.
DataSyteAiCLS is a significant improvement over existing apparatus for performing resuscitation procedures. Unlike traditional carts, which require manual input from the response team, DataSyteAiCLS requires minimal input and can automatically guide the response team through the procedure. This device is a crucial tool for any medical facility that wants to increase the efficiency and effectiveness of its resuscitation procedures.
SUMMARY OF THE INVENTION
The main focus of the current innovation is to introduce DataSyteAiCLSI that can assist the hospital response team in carrying out cardiopulmonary resuscitation. The purpose of this innovation is to address several objectives that enhance the effectiveness and efficiency of the response team during CPR.
One of the objectives is to enable DataSyteAiCLS to convey and dispense medical supplies. Another goal is to reduce the occurrence of human errors and knowledge gaps that could impede the success of the CPR procedure. The innovation also aims to improve the efficiency of the response team during CPR.
Additionally, DataSyteAiCLS is designed to calculate doses accurately and requires minimal input from the response team during the CPR process. It can retrieve patient records and update them with the details of the intervention, as well as retrieve patent diagnostic/monitoring data from the patient monitoring system.
DataSyteAiCLS also facilitates the response team’s ability to locate medicine and equipment arranged within the apparatus. It records the CPR procedure for later analysis and training purposes and provides visible and audible alarms for various unsafe conditions. Moreover, the innovation offers simulated training of ACLS protocols and maintains a running inventory of the medical supplies contained in the cart.
Finally, DataSyteAiCLS provides audible instructions to the response team. The ensuing description and accompanying drawings will provide further details on the objectives and benefits of this innovation.
Claims
Assisting response teams during cardiopulmonary resuscitation (CPR) can be a challenging task, but DataSyteAiCLS helps streamline the process. DataSyteAiCLS includes a control unit, memory, and multiple drawers containing medical equipment and medicines. It also has cameras, a display, and input devices to provide a comprehensive solution.
DataSyteAiCLS receives patient details and identifies the standard protocol for performing CPR. It then analyzes a sequence of images from the cameras to recognize actions and gestures of the response team. Based on this analysis, DataSyteAiCLS provides step-by-step instructions to the team and observes any errors in following the standard protocol. If necessary, it issues alerts to the team based on the error.
Patient details can be retrieved from a database or provided by the attendants. DataSyteAiCLS can calculate the intervention dose based on the patient’s weight. It can also display a list of standard protocols and allow the team to select one based on the patient’s condition.
The standard protocol is based on ACLS guidelines. DataSyteAiCLS can update the patient record with details of interventions admitted to the patient. It can receive details of withdrawal of an intended medicine from one of the drawers and corroborate it with the action of the administration of the intended medicine.
DataSyteAiCLS guides the response team to the correct drawer by blinking an indicator and opening the correct drawer. It can also store the video in the memory and display step-by-step instructions on the display and broadcast them through the speaker.
DataSyteAiCLS retrieves data from the medical equipment related to the patient’s condition. It includes sensors to detect withdrawal of medicines and indicators to blink to guide the team to the correct drawer. It also includes a touch input interface, a microphone to interpret voice commands, and RFID-based sensors.
In conclusion, DataSyteAiCLS is a valuable asset in assisting response teams during CPR. It provides step-by-step instructions, identifies errors, and issues alerts.
PathFree Technologies Corporation’s brand AiMediQ boasts a plethora of ingenious medical devices that are currently in their final developmental stages, and are soon to be manufactured and made available to the global medical community. Among these products, one in particular stands out – DataSyteAiCLS. This groundbreaking medical product has never been available before, and we anticipate that it will soon become the new standard for all doctors performing cardiopulmonary arrest procedures.
Our team has worked diligently to create this patented and protected device, which is now on the cusp of being made accessible to medical professionals worldwide. With its innovative design and unique features, DataSyteAiCLS promises to change the landscape of the way cardiopulmonary arrest procedures are performed, making the process safer and more efficient than ever before.
Artificial Intelligence (AI)
We have incorporated AI to significantly improve DataSyteAiCLS in various ways, enhancing its features and helping medical response teams perform even more effectively during emergency situations:
Advanced Machine Learning Algorithms: We have Integrated more sophisticated AI algorithms into DataSyteAiCLS system allows for faster and more accurate identification of the appropriate ACLS protocols based on the patient’s condition. These algorithms can also improve real-time analysis and feedback during resuscitation procedures, leading to better patient outcomes.
Natural Language Processing (NLP): We have Incorporated NLP technology which has enabled DataSyteAiCLS to understand and interpret spoken instructions from the response team, allowing for more seamless communication and reducing the need for manual input.
Predictive Analytics: DataSyteAiCLS uses predictive analytics to forecast potential complications or changes in a patient’s condition during a resuscitation procedure. This can help the response team make informed decisions and adjust their approach proactively, resulting in improved patient care.
Integration with IoT Devices: Connecting DataSyteAiCLS to other Internet of Things (IoT) medical devices, such as patient monitoring systems and wearable sensors, can allow the AiMediQ AiCart to collect and analyze more comprehensive data on a patient’s condition in real-time. This can further improve the accuracy and effectiveness of DataSyteAiCLS’ recommendations and guidance.
Enhanced Training and Simulation: DataSyteAiCLS helps create more realistic and immersive training simulations for medical response teams, incorporating real-world scenarios and varying levels of difficulty to better prepare healthcare professionals for emergency situations.
Automatic Inventory Management: AI optimizes the management of medical supplies within DataSyteAiCLS, ensuring that essential equipment and medications are always in stock and ready for use. This can help prevent shortages and reduce the risk of delays during critical resuscitation procedures.
Continuous Improvement: DataSyteAiCLS analyzes data collected from past resuscitation procedures and identify patterns or trends that can inform improvements in DataSyteAiCLS’ functionality, as well as help refine ACLS protocols and best practices.
With these integrated AI-driven features and capabilities, DataSyteAiCLS has become an even more powerful tool for medical response teams, further reducing knowledge gaps, minimizing human errors, and ultimately improving patient outcomes during cardiopulmonary arrest situations.
We are confident that this product will be highly sought-after by doctors and medical institutions alike, and we are excited to bring it to the market. Our commitment to providing cutting-edge medical technology that improves patient outcomes is unwavering, and we are proud to be at the forefront of innovation in the healthcare industry.

AiMediQ MediQ(PathResponder): The Essential ACLS Companion for Pre-Hospital Care
Empowering Emergency Response with Compact Intelligence:
AiMediQ MediQ(PathResponder) isn’t just a medical device; it’s a lifeline for healthcare professionals in pre-hospital settings. This compact and portable system serves as the core platform for PathFree’s solutions, equipping medical teams with everything they need to manage critical ACLS situations effectively.
Why It Matters to Equity Investors:
Addressing a Growing Market: The demand for advanced pre-hospital care is rising, driven by aging populations and increasingly complex medical emergencies. AiMediQ MediQ offers a solution that fills a critical gap, making it a highly attractive investment opportunity.
Unmatched ACLS Support: AiMediQ MediQ boasts:
Real-time vital sign monitoring: Keep track of patients’ vital signs with continuous monitoring and data visualization.
Onboard diagnostic tools: Perform essential checks like ECG, pulse oximetry, and blood pressure measurements to inform treatment decisions.
Secure communication capabilities: Stay connected with hospitals and specialists for seamless transfer and consultation.
Rugged and portable design: Built for the harshest conditions, AiMediQ MediQ thrives in ambulances, helicopters, and challenging environments.
Data-Driven Insights: Like AiMediQ AiCart, MediQ collects valuable data on ACLS procedures, enabling continuous improvement and data-backed decision-making. This data further strengthens your case for investors.
Scalability and Accessibility: AiMediQ MediQ’s compact size and modular design make it adaptable to various pre-hospital settings, from large ambulance fleets to small first-responder vehicles. This broadens its market reach and potential return on investment.
The MediQ has the potential to:
Save lives: By improving ACLS efficiency and accuracy, AiMediQ MediQ can directly impact patient survival rates.
Optimize resource allocation: Accurate data insights can guide strategic decisions and improve resource allocation within healthcare systems.
Empower pre-hospital teams: Providing first responders with the tools they need to make informed decisions improves confidence and performance in critical situations.
PathFree Technologies’ AiMediQ MediQ (PathResponder) represents a significant advancement in pre-hospital emergency care, particularly in Advanced Cardiovascular Life Support (ACLS) situations. This device, designed to be compact and portable, is specifically tailored for use in pre-hospital settings like ambulances and emergency response vehicles. It offers a range of functionalities that address the growing demand for advanced pre-hospital care driven by an aging population and increasingly complex medical emergencies.
The AiMediQ MediQ (PathResponder) is equipped with capabilities crucial for effective ACLS support in pre-hospital environments. These include real-time monitoring of vital signs, onboard diagnostic tools for essential checks such as ECG, pulse oximetry, and blood pressure measurements, and secure communication features to ensure seamless coordination with hospitals and specialists. Its rugged and portable design makes it ideal for use in various challenging conditions.
In addition to its practical features, the AiMediQ MediQ (PathResponder) collects valuable data on ACLS procedures, similar to the AiMediQ AiCart. This data collection is pivotal for continuous improvement and informed decision-making, which is particularly attractive to equity investors. The device’s scalable and adaptable design allows it to fit different pre-hospital settings, enhancing its market reach and potential investment returns.
PathFree Technologies emphasizes the use of Artificial Intelligence (AI) in their products, including the AiMediQ MediQ (PathResponder), to enhance their effectiveness in emergency situations. The integration of AI in these devices is seen as a step forward in revolutionizing emergency care, potentially improving patient survival rates and optimizing healthcare resources. The company’s focus on AI-driven solutions, like the AiMediQ AiCart, has been geared towards reducing knowledge gaps, minimizing human errors, and enhancing the efficiency of medical response teams in cardiopulmonary arrest situations.

AiMediQ MobileER (PathEmergency): The Ultimate ACLS Fortress for Mass Casualty Events
When Every Second Counts, There’s AiMediQ MobileER:
Imagine a self-contained, rapidly deployable medical unit, equipped with cutting-edge ACLS technology and capable of transforming into a fully functional treatment environment within minutes. That’s AiMediQ MobileER (PathEmergency) – the future of emergency response for large-scale events and disasters.
Why It Matters to Equity Investors:
Addressing a Critical Gap: Mass casualty events, from natural disasters to terrorist attacks, strain existing healthcare systems. AiMediQ MobileER offers a solution by providing immediate, high-quality ACLS care to a large number of patients simultaneously.
Unmatched ACLS Capability: AiMediQ MobileER boasts:
Modular design: Expandable units adapt to the scale of any emergency, ensuring efficient patient flow and treatment.
Advanced ACLS technology: Integrate AiMediQ’s core functionalities with additional medical equipment for comprehensive critical care.
Precision drone support: Digital twin technology guides medical drones for rapid aerial transportation, medication delivery, and patient triage.
Secure communication infrastructure: Seamlessly connect with hospitals and emergency networks for coordinated response and resource allocation.
Data-Driven Insights and Improvement: Real-time data gathering and analysis inform decision-making, optimize workflows, and ensure continuous improvement in disaster response.
Proven Technology and Leadership: Built on PathFree’s proven AiMediQ platform and backed by experienced leadership.
Global Market Impact: From earthquake zones to conflict areas, AiMediQ MobileER has the potential to save lives and improve emergency response worldwide, leading to substantial market reach and return on investment.
AiMediQ MobileER can:
Minimize fatalities: Rapid deployment and advanced ACLS capabilities save lives during critical hours after disasters.
Optimize resource allocation: Data-driven insights guide deployment and resource allocation, maximizing impact with limited resources.
PathFree Technologies’ AiMediQ MobileER (PathEmergency) is a groundbreaking development in the field of emergency response, especially for handling mass casualty events. This innovative device is engineered with the advanced features of PathFree’s AiMediQ technology, tailored for civilian use in emergency scenarios. Its key characteristics make it highly relevant for equity investors who are interested in the healthcare and emergency response sectors.
The AiMediQ MobileER offers a modular design, which allows it to adapt to various scales of emergencies, ensuring efficient patient flow and treatment. It integrates core functionalities of the AiMediQ platform with additional medical equipment, providing comprehensive critical care. One of the most notable features of the AiMediQ MobileER is its precision drone support, which utilizes digital twin technology for rapid aerial transportation, medication delivery, and patient triage. This feature enhances its capability to provide immediate, high-quality ACLS care to a large number of patients simultaneously.
The device is also equipped with a secure communication infrastructure, allowing seamless connectivity with hospitals and emergency networks for coordinated responses and resource allocation. In terms of data management, the AiMediQ MobileER gathers and analyzes real-time data to inform decision-making, optimize workflows, and ensure continuous improvement in disaster response scenarios.
PathFree Technologies’ brand AiMediQ emphasizes the integration of Artificial Intelligence (AI) and machine learning in their products, including the AiMediQ MobileER. AI in healthcare, particularly in critical care, is transformative, enabling predictive analytics, pattern recognition, and data-driven research that significantly improve patient outcomes and healthcare efficiency. The AiMediQ MobileER, like other AiMediQ products, is designed for continuous improvement, with AI capabilities allowing it to learn and adapt over time, thereby enhancing its effectiveness in emergency situations.
The combination of these advanced technologies and the device’s portability make it a valuable asset in emergency medical response, particularly in remote or challenging terrains and in situations where conventional medical response methods might be insufficient or too slow. The potential lifesaving impact of the AiMediQ MobileER is significant, especially in large-scale emergencies or mass casualty events where rapid deployment and advanced medical capabilities can dramatically reduce mortality rates.

AiMediQ AiMini (PathEvent): The Backpack-Sized Powerhouse for Event ACLS Preparedness
Safeguarding Crowds with Cutting-Edge Technology:
Large gatherings come with risks, and when it comes to ACLS preparedness, every second counts.
AiMediQ AiMini (PathEvent) – the compact, on-the-go solution for ensuring swift and effective response during potential medical emergencies at crowded events.
Why It Matters to Equity Investors:
Addressing a Growing Concern: Event safety is paramount, and AiMediQ AiMini tackles the rising concern of managing potential ACLS situations at concerts, sporting events, and other large gatherings.
Unmatched ACLS Readiness: AiMediQ AiMini boasts:
Essential vital sign monitoring: Track patients’ vitals in real-time and identify potential issues before they escalate.
Secure communication capabilities: Stay connected with medical teams and emergency responders for seamless coordination.
Digital twin training simulations: Utilize AiMediQ AiMini’s technology to train medical professionals on ACLS protocols and improve response preparedness.
Compact and portable design: Easily carried by event staff, security personnel, or even first responders for immediate deployment.
Data-Driven Insights and Improvement: Gather valuable data on event medical responses to refine protocols and optimize future preparedness.
Scalability and Accessibility: AiMediQ AiMini’s affordability and ease of use make it accessible to event organizers of all sizes, democratizing ACLS preparedness and safety standards.
A Proven Technology Legacy: Built on PathFree’s established AiMediQ platform, AiMediQ AiMini leverages trusted technology and expertise, making it a reliable investment.
Invest in Protecting Crowds, One Event at a Time:
By backing AiMediQ AiMini, you’re not just investing in a device; you’re investing in peace of mind and security for millions of people attending events worldwide. AiMediQ AiMini can:
Minimize panic and chaos: Rapid response and communication during medical emergencies prevent escalation and maintain a safe environment.
Empower event staff and first responders: Equip them with the tools and confidence to handle potential ACLS situations effectively.
Promote preventative healthcare: Early identification of potential issues through vital sign monitoring allows for proactive intervention and avoids escalation.
PathFree Technologies’ AiMediQ AiMini (PathEvent) is an innovative product tailored for providing ACLS support in crowded event settings. It represents a compact, portable solution designed to enhance safety and medical preparedness at large gatherings, such as concerts and sports events.
The AiMediQ AiMini incorporates essential features for ACLS readiness. It includes capabilities for real-time vital sign monitoring, which is crucial in quickly identifying and responding to potential medical issues before they escalate. Additionally, its secure communication capabilities ensure seamless coordination with medical teams and emergency responders, enhancing the efficiency of medical interventions during events.
One of the notable aspects of AiMediQ AiMini is its use of digital twin technology for training simulations. This technology helps in training medical professionals on ACLS protocols, thereby improving response preparedness for potential emergencies. Its compact and portable design makes it easily accessible and deployable by event staff, security personnel, or first responders, ensuring immediate action during emergencies.
PathFree Technologies’ brand AiMediQ emphasizes the integration of Artificial Intelligence (AI) and machine learning in its products, including AiMediQ AiMini. This integration facilitates enhanced medical response capabilities, such as predictive analytics for risk factor detection and proactive care, and pattern recognition for more accurate diagnoses and treatment plans. The AI features in AiMediQ AiMini and other AiMediQ products are designed to complement human expertise in healthcare, providing continuous monitoring and data analysis to improve patient outcomes.
See Our Story On Fox News

PathFree Wrist-mounted Aerosol Vacuum Filter
Click Here To See Patent Information
Introduction
Reducing Pathogen Load with the PathFree Wrist-mounted Aerosol Vacuum Filter Assembly in the Medical Field
In healthcare settings, patient safety is paramount, and reducing the risk of infections is a top priority. The COVID-19 pandemic has shown us how easily pathogens can spread through the air, which is why there is a need for a device that can limit the spread of microbes and decrease the pathogen load near the mouth of an infected person. The solution comes in the form of a miniature air filtration assembly that can reduce the pathogen load in an airway of a patient during a medical procedure or while wearing an oxygen mask.
Introducing the PathFree Wrist-mounted Aerosol Vacuum Filter Assembly
The PathFree Wrist-mounted Aerosol Vacuum Filter assembly is a novel invention designed to reduce the microbial load in a patient’s airway. It includes a miniature vacuum unit that measures 1 cubic inch in size and is cubical in shape. The vacuum unit comprises a housing, at least one air inlet for air intake, a vacuum motor for sucking air through the air inlet, vents for blowing the sucked air out of the housing, and a filter media covering the inner side of the vents. The filter media is configured to retain microbes suspended in the sucked air.
Additionally, the assembly includes at least one suction tube that has a proximal and a distal end. The proximal end of the suction tube is sealably and releasably coupled to the air inlet, and a plurality of apertures are configured in the wall of the suction tube near its distal end. The suction tube is positioned within the mouth of the patient to suck air through the apertures. The sucked air passes through the filter media, which retains any microbes suspended in the air. The assembly may also have a UV lamp enclosed in the housing to irradiate the filter media.
Advantages of the PathFree Wrist-mounted Aerosol Vacuum Filter Assembly
The PathFree Wrist-mounted Aerosol Vacuum Filter assembly provides several benefits that make it a compelling solution for reducing pathogen load in the medical field. First, it is designed to be used with known medical devices, such as a conventional laryngoscope, and does not interfere with medical procedures or cause discomfort to the patient. Which makes it easy to use and economical to manufacture. Second, it decreases the chances of healthcare workers acquiring an infection during a medical procedure, providing additional protection to medical staff.
Method of Using the PathFree Wrist-mounted Aerosol Vacuum Filter Assembly
To use the PathFree Wrist-mounted Aerosol Vacuum Filter assembly, the suction tube is positioned within the patient’s mouth, and air is sucked through the apertures of the suction tube. In some cases, there may be two air inlets and two suction tubes, with one suction tube inserted into the oxygen mask and the other suction tube wrapped around the mask. This method ensures that the air in the patient’s airway is filtered, reducing the risk of spreading pathogens.
The Benefits of the PathFree Wrist-mounted Aerosol Vacuum Filter Assembly in the Medical Field
The PathFree Wrist-mounted Aerosol Vacuum Filter assembly offers several advantages in the medical field. One of the most significant benefits is that it helps to reduce the risk of spreading pathogens during medical procedures. The assembly is specifically designed to filter the air in a patient’s airway, reducing the pathogen load and improving patient safety. The miniature size of the assembly makes it easy to handle and use, and it is also economical to manufacture. Additionally, the assembly is designed not to interfere with medical procedures, which is crucial for ensuring that patients receive the best possible care.
Using the PathFree Wrist-mounted Aerosol Vacuum Filter Assembly in Various Medical Settings
The PathFree Wrist-mounted Aerosol Vacuum Filter assembly can be used in various medical settings to reduce the spread of pathogens. For example, it can be used in emergency rooms, surgical centers, and intensive care units. The assembly is particularly useful in situations where patients are suspected or confirmed to have a contagious disease, such as COVID-19 or influenza. It is also useful for procedures that involve intubation, such as tracheal intubation, where the risk of airborne transmission is high. The PathFree Wrist-mounted Aerosol Vacuum Filter assembly can be used in combination with other personal protective equipment, such as gloves, masks, and gowns, to further reduce the risk of infection transmission.
Advancements in the Medical Field with the PathFree Wrist-mounted Aerosol Vacuum Filter Assembly
The PathFree Wrist-mounted Aerosol Vacuum Filter assembly represents a significant advancement in the medical field. It offers a novel solution to a problem that has plagued healthcare workers for years – the risk of acquiring infections while performing medical procedures. The assembly’s unique design and ease of use make it a valuable addition to any healthcare facility, and ensures that it remains hygienic and free from contamination.
Future Developments of the PathFree Wrist-mounted Aerosol Vacuum Filter Assembly
As technology advances, so too will the PathFree Wrist-mounted Aerosol Vacuum Filter assembly. It is likely that future iterations of the assembly will be even smaller and more efficient, making them even more useful in a variety of medical settings. Additionally, further research may reveal additional benefits of using the assembly, such as its effectiveness in reducing the spread of other types of airborne diseases.
Conclusion
In conclusion, the PathFree Wrist-mounted Aerosol Vacuum Filter assembly is a valuable tool in reducing the pathogen load near the mouth of an infected person during medical procedures. Its unique design and ease of use make it a compelling solution for healthcare workers looking to improve patient safety and reduce the risk of infection transmission. As advancements in technology continue, it is likely that the assembly will become even more efficient and useful in various medical settings. Overall, the PathFree Wrist-mounted Aerosol Vacuum Filter assembly is an innovative and necessary addition to any healthcare facility, providing an extra layer of protection to patients and healthcare workers alike.
We are confident that this product will be highly sought-after by doctors and medical institutions alike, and we are excited to bring it to the market. Our commitment to providing cutting-edge medical technology that improves patient outcomes is unwavering, and we are proud to be at the forefront of innovation in the healthcare industry.
PathFree Expandable Endotracheal Tube
Introducing the PathFree Expandable Endotracheal Tube – a revolutionary medical device that enables the gradual increase of the internal diameter of an endotracheal tube without the need for replacement. The endotracheal tube is a flexible plastic tube commonly used in emergency medicine, critical care, mechanical ventilation, and surgery with anesthesia to establish and maintain a patent airway.
However, selecting the correct size of the tube for a patient is crucial, as the use of an incorrect size may cause complications. A smaller diameter tube is safer and easier to insert, but it is more likely to be obstructed by secretions, while a larger size tube can permit other devices to pass through it more easily. Unfortunately, the process of replacing the tube can be complex and life-threatening.
The PathFree Expandable Endotracheal Tube solves this problem with its unique design. It comprises a tunnel configured into the wall of the endotracheal tube, which can be expanded to increase the circumference and thus, the internal diameter of the tube. The tunnel can replaceably receive an expander of different widths, which gradually increases the internal diameter of the endotracheal tube without removing it from the trachea.
The endotracheal tube assembly is economical to manufacture and is made of stretchable material, making it flexible and easy to use. The expander is made of a flexible material and has an arc-shaped elongated body with a blunted tip for easy insertion into the tunnel. The width of the expander is proportional to the increase in the circumference of the endotracheal tube.
The method of using the PathFree Expandable Endotracheal Tube is simple. The endotracheal tube is first inserted into the trachea, and then the expander of the first width is inserted into the tunnel through the opening at the proximal end of the endotracheal tube, increasing the internal diameter of the tube proportionally to the width of the expander. If necessary, the expander can be replaced with another of a different width to further adjust the internal diameter of the endotracheal tube.
In conclusion, the PathFree Expandable Endotracheal Tube is a game-changing medical device that enables the gradual increase of the internal diameter of an endotracheal tube without the need for replacement, avoiding the potential complications of tube replacement.
BACKGROUND
An endotracheal tube is a medical device utilized to establish and maintain an open airway in patients by inserting a flexible plastic tube through the mouth and into the trachea. This tube is commonly used in emergency medicine, critical care, mechanical ventilation, and anesthesia during surgery. The process of inserting the tube is called endotracheal intubation.
Endotracheal tubes come in various sizes, ranging from 2.0 millimeters to 12.0 mm internal diameters in 0.5 mm increments. The appropriate size of the tube is important and differs according to the patient’s gender and age. Generally, a 7.0 to 7.5 mm diameter tube is often used for females, while an 8.0 to 9.0 mm diameter tube is used for males. Newborns require a 3.0 mm to 3.5 mm tube, with a 2.5 to 3.0 mm tube for premature infants.
Smaller tubes are easier to insert and result in a lower incidence of sore throat after intubation, making them safer for the trachea. Additionally, the smaller diameter of the tube allows for a better view of the larynx during insertion, resulting in less trauma to the patient. However, small diameter tubes are more prone to obstruction by secretions, which can cause resistance and obstruction to gas flow. On the other hand, larger tubes have certain advantages, such as allowing other devices to pass through them easily and reduced driving pressures. Nevertheless, doctors must choose the appropriate size of the tube for each patient.
Choosing the wrong size of the tube may lead to complications, such as esophageal intubation, loss of the airway, severe hypoxia, or cardiac arrest. Therefore, there is a need for an improved endotracheal tube with an adjustable internal diameter that can be controlled in each millimeter unit. This innovation would prevent the need to replace the tube and mitigate the risks and costs associated with the replacement procedure.
In conclusion, endotracheal tubes are essential medical devices in emergency medicine, critical care, mechanical ventilation, and anesthesia during surgery. The appropriate size of the tube is crucial for the safety and wellbeing of the patient. Therefore, it is essential to have an improved endotracheal tube with a variable internal diameter to avoid complications associated with replacing the tube.
SUMMARY
In this section, we will provide a simplified summary of the invention, outlining its main objectives and components. This summary aims to provide a basic understanding of the invention and is not intended to identify critical elements or scope. Its sole purpose is to introduce the concepts of the invention before delving into a more detailed description later.
The primary objective of the invention is to improve endotracheal tube assemblies by providing an endotracheal tube with a gradually increasing internal diameter, thus avoiding the need to replace the tube with a larger size. Another objective is to create an economically viable endotracheal tube assembly.
The invention consists of an endotracheal tube assembly, comprising an endotracheal tube and an expander. The endotracheal tube has a tubular body with a proximal end, a distal end, and a wall extending between them. The proximal end can be connected to a medical apparatus, while the distal end is inserted into the trachea. The wall contains one or more tunnels that can be expanded, increasing the tube’s internal diameter. The tunnel is open at the proximal end and closed at the distal end.
The expander has an arc-shaped elongated body with a handle at one end and a blunted tip at the other. It is made of a flexible material and inserted into the tunnel, with the blunted tip expanding the tunnel. The expander comes in different widths, and the width of the expander is proportional to the change in the internal diameter of the endotracheal tube.
To insert the endotracheal tube, it is inserted through the mouth into the trachea, with the distal end going in first. After the tube is inserted, an expander of a certain width is inserted into the tunnel through the opening of the tunnel at the proximal end. This insertion increases the internal diameter of the endotracheal tube proportionally to the width of the expander.
To adjust the internal diameter of the endotracheal tube, the expander of the initial width is removed from the tunnel, and an expander of a different width is inserted. This new expander can have a width less than or greater than the initial expander, adjusting the internal diameter proportionally.
In conclusion, the invention presents an improved endotracheal tube assembly that eliminates the need for larger tubes and provides economic benefits. It consists of an endotracheal tube and an expander that can increase or decrease the tube’s internal diameter. We hope this summary has provided a basic understanding of the invention’s concepts.
Claims
The PathFree Expandable endotracheal tube assembly is a medical device used for inserting and securing an endotracheal tube in a patient’s trachea. The assembly comprises an endotracheal tube with a proximal end, a distal end, and a wall in between the two ends. The wall is interrupted by a tunnel that extends between the proximal and distal ends of the tube. The tunnel is designed to expand and increase the circumference of the endotracheal tube when required.
The expander used to expand the tunnel has an elongated body and can be removably received and retained into the tunnel. The width of the expander is proportional to the increase in the circumference of the endotracheal tube. The proximal end of the tube can be fluidly connected to a medical device, while the distal end is designed for insertion into the patient’s trachea. The tube’s proximal end can be coupled to a connector that is attached to the medical device.
The tunnel is sealed at the distal end and has an opening at the proximal end. The opening can be closed during insertion into the trachea and opened when inserting the expander. The endotracheal tube’s distal end is beveled to ease its insertion into the trachea. The tube also has an inflatable cuff near its distal end and at least one murphy eye adjacent to the distal end.
The tunnel is made of one or more layers of a stretchable material, and the joint between the tunnel and the wall is sealed by a stretchable lining. To perform endotracheal intubation using this assembly, the endotracheal tube assembly is provided, and the tube is inserted into the trachea. The expander is then inserted into the tunnel to increase the tube’s circumference.
The method can also include inserting an expander of a different width and removing the previous one. The tunnel’s opening is switched between open and closed positions, and one end of the expander is blunted for insertion into the tunnel.
PathFree Technologies Corporation boasts a plethora of ingenious medical devices that are currently in their final developmental stages, and are soon to be manufactured and made available to the global medical community. Among these products, one in particular stands out – the PathFree Expandable Endotracheal Tube. This groundbreaking medical product has never been available before, and we anticipate that it will soon become the new standard for all doctors performing intubation procedures.
Our team has worked diligently to create this patented and protected device, which is now on the cusp of being made accessible to medical professionals worldwide. With its innovative design and unique features, the PathFree Expandable Endotracheal Tube promises to revolutionize the way intubation procedures are performed, making the process safer and more efficient than ever before.
We are confident that this product will be highly sought-after by doctors and medical institutions alike, and we are excited to bring it to the market. Our commitment to providing cutting-edge medical technology that improves patient outcomes is unwavering, and we are proud to be at the forefront of innovation in the healthcare industry.
PathFree Septum Monitor
Introducing the PathFree Septum Monitor, a cutting-edge monitor equipment that improves electro-surgical unit (ESU) and vital signs (VS) monitoring during surgeries. This device comprises a nasal clip that attaches to the patient’s nasal septum, a monitor detector that connects to the nasal clip via a wired or wireless connection, and an ESU that connects to the monitor detector via a wired or wireless connection.
In modern surgeries, electronic devices, such as the ESU, have become a routine equipment item to reduce blood loss and enable a more rapid recovery. However, monitoring vital signs can be vulnerable to unintentional interference from ESU and human activity by the patient or surgical staff. Moreover, concerns have been raised in the medical community regarding electronic medical records, medical research, and clinic quality management.
To address these concerns, the PathFree Septum Monitor was developed to provide accurate and reliable VS monitoring during surgical procedures. The nasal clip comprises two probes that are inserted into the patient’s nasal cavities, with the ends of the probes touching the surface of the nasal septum. At least one side of a probe includes either a temperature sensor or a part of a pulse oximeter (POx) sensor.
The ESU includes a generator, a handpiece, and a foot switch, with the on/off switch located on either the handpiece or the foot switch. An internal switch is also activated when the on/off switch is activated. The internal switch is configured to send an image or a signal from the generator to the monitor detector. The monitor detector displays the signal differently from any other information displayed on the monitor detector, using a differently colored waveform or screen background.
The PathFree Septum Monitor also includes a motion sensor that is placed on the patient’s chest and is connected to the ESU. This motion sensor is designed to detect outside interference during the surgical procedure. Additionally, a cuff shell can be placed over a sphygmomanometer cuff on the patient’s arm, with pressure sensors that detect any external pressure applied to the cuff shell. The cuff shell also includes a hardware extension to prevent any external pressure from being applied to the patient’s arm.
A scanner is also included in the PathFree Septum Monitor, which is configured to scan a medication label on a syringe. Using image recognition, the scanner measures the volume changes of the dosage in the syringe and calculates the dosage injected based on the measured volume changes. The scanner also includes a display device to show the dosage injected and a communication device that can transmit a signal related to the dosage injected or injection time to a user device.
In conclusion, the PathFree Septum Monitor is a sophisticated and innovative monitor equipment that overcomes the limitations and problems associated with VS monitoring during surgical procedures. It provides accurate and reliable monitoring that is critical for patient safety and well-being.
BACKGROUND OF THE INVENTION
Modern surgery relies heavily on electronic devices. In surgeries to cut, coagulate, dissect, fulgurate, ablate, and decrease tissue, the electro-surgical unit (ESU) has become a routine equipment item to reduce blood loss, which leads to more rapid recovery. However, the vital signs (VS) monitoring is vulnerable to unintentional interference from ESU and human activity by the patient or the surgical staff. Furthermore, concerns have been raised in the medical community regarding electric medical records and medical research, as well as clinic quality management.
For example, during new drug clinical research on surgical patients, copy-and-paste or record cloning can be done from standard guideline or protocols. A missed edit from “positive result” to “negative result” can have devastating effects on not only a patient’s record, but also on the patient’s treatment and the reports of medical researchers as well as quality control of the research. For example, during a clinical observation of a new drug administration during a surgery, on the monitor screen, a patient’s significant decline in blood pressure with arrhythmia after the physician administered the new drug about one minute later, at the same time the ESU was active or others, can support a management decision which can be made after the ESU is deactivated, which will obtain a reliable record for study and review.
Accordingly, there is a need for an improved surgical equipment and VS monitor that may overcome one or more of the abovementioned problems and/or limitations.
SUMMARY OF THE INVENTION
This summary is provided to introduce a selection of concepts in a simplified form that are further described below in the Detailed Description. This summary is not intended to identify key features or essential features of the claimed subject matter. Nor is this summary intended to be used to limit the claimed subject matter’s scope.
According to some embodiments, the present disclosure relates to a monitor equipment. The monitor equipment comprising a nasal clip configured to clip onto nasal septum of a patient. Further, the monitor equipment comprising a monitor detector connected to the nasal clip through at least one of a wired and a wireless connection. The monitor detector may be a vital signs (VS) monitor. Further, the monitor equipment comprising an electro-surgical unit (ESU) connected to the monitor detector through at least one of a wired and a wireless connection.
Both the foregoing summary and the following detailed description provide examples and are explanatory only. Accordingly, the foregoing summary and the following detailed description should not be considered to be restrictive. Further, features or variations may be provided in addition to those set forth herein. For example, embodiments may be directed to various feature combinations and sub-combinations described in the detailed description.
Claims
Introducing a state-of-the-art medical device that comprises several components to help healthcare providers monitor their patients with greater accuracy and efficiency. The device consists of a nasal clip designed to clip onto the nasal septum of the patient, a monitor detector connected to the nasal clip through a wired or wireless connection, and an electro-surgical unit (ESU) also connected to the monitor detector via a wired or wireless connection.
The nasal clip features two probes that are inserted into the patient’s nasal cavities, with their ends touching the surface of the nasal septum to provide precise monitoring data. Additionally, at least one side of the probes includes either a temperature sensor or a pulse oximeter (POx) sensor to record relevant patient data accurately.
The ESU component comprises a generator, a handpiece, and a foot switch, with at least one of these elements featuring an on/off switch to control the device. Furthermore, the handpiece and foot switch include an internal switch that activates whenever the on/off switch is activated. This internal switch sends an image or signal from the generator to the monitor detector, which displays the information differently from other data on the device, utilizing different colored waveforms or screen backgrounds.
To further enhance the monitoring process, the device also includes a motion sensor placed on the patient’s chest to detect any outside interference. Moreover, there is a cuff shell placed over a sphygmomanometer cuff that includes pressure sensors to detect any external pressure applied to the cuff shell. The cuff shell also has a hardware extension to prevent any external pressure from being applied to the patient’s arm.
Finally, the device includes a scanner designed to scan medication labels on a syringe. The scanner uses image recognition technology to measure volume changes in the dosage and calculate the dosage injected based on the measured volume changes. The scanner includes a display device to show the dosage injected and a communication device that transmits a signal related to the dosage injected or injection time to a user device.
In conclusion, this medical device is a cutting-edge solution to improve patient monitoring and provide healthcare providers with more accurate and efficient tools.
PathFree Technologies Corporation boasts a plethora of ingenious medical devices that are currently in their final developmental stages, and are soon to be manufactured and made available to the global medical community. Among these products, one in particular stands out – the PathFree Septum Monitor. This groundbreaking medical product has never been available before, and we anticipate that it will soon become the new standard for all doctors performing modern surgery procedures.
Our team has worked diligently to create this patented and protected device, which is now on the cusp of being made accessible to medical professionals worldwide. With its innovative design and unique features, the PathFree Septum Monitor promises to revolutionize the way modern surgery procedures are performed, making the process safer and more efficient than ever before.
We are confident that this product will be highly sought-after by doctors and medical institutions alike, and we are excited to bring it to the market. Our commitment to providing cutting-edge medical technology that improves patient outcomes is unwavering, and we are proud to be at the forefront of innovation in the healthcare industry.
Reinventing Emergency Response: The AiMediQ MediQ Drone Revolution

Intelligent Response Unit
Artificial Intelligence
Machine Learning
Predictive Analytics
Enhanced Training & Simulation
Automatic Inventory Management
Connects With Internet Of Things
Natural Language Processing
Drone-capable
AiMini / AiMediQ
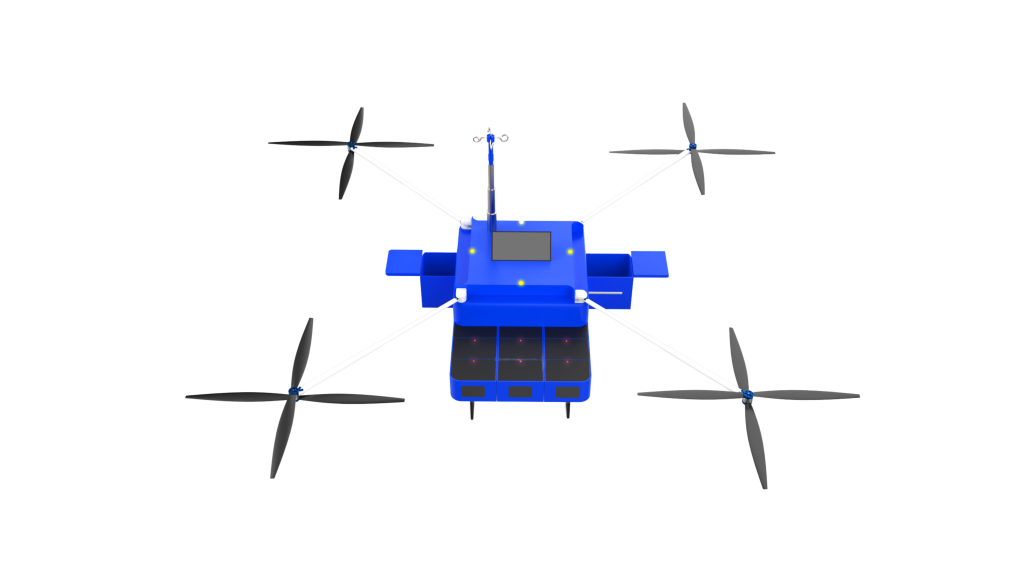
In the realm of rapid emergency response, the intersection of Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), and predictive analytics is paving the way for transformative innovations. At the forefront of these changes is the prospect of transforming the AiMediQ AiCart into a mini drone-able device. This innovative blend of technologies could overhaul the way healthcare decisions are made and redefine emergency responses.
Rapid and Unhindered Response
One of the critical benefits of drone-enabled AiMediQ technology is the speed at which help can reach those in need. Unlike traditional emergency services vehicles, drones can bypass the constraints of traffic, leading to a rapid response that can be crucial during emergencies. This capability to reach a destination quickly can make a significant difference in life-threatening situations.

Extending Reach to Remote Areas
Drones equipped with AiMediQ technology hold the potential to provide remote accessibility. They can easily reach areas where traditional emergency services might struggle, significantly expanding the reach of critical care and ensuring no location is left isolated during crises.
Real-Time Monitoring and Data Analysis
By integrating with the IoT and utilizing real-time data, drones can provide real-time monitoring. They can relay vital patient information back to medical professionals while en route, enabling immediate analysis and pre-arrival preparation at the patient’s location. This feature ensures that healthcare professionals can start planning the treatment even before reaching the scene.
Efficient Delivery of Medical Supplies
Another key function of these drone-able AiMediQ MediQs is the efficient delivery of medical supplies. This function becomes particularly vital in situations where immediate human response isn’t feasible or when the patient is in a remote location.
Cost-Effectiveness and Sustainability
Drone deployment also provides a cost-effective alternative to traditional methods. By reducing reliance on ambulances and other emergency vehicles, healthcare systems can cut down on costs significantly. Furthermore, as most drones are electric, they can also align with environmental sustainability goals, contributing to a reduced carbon footprint.
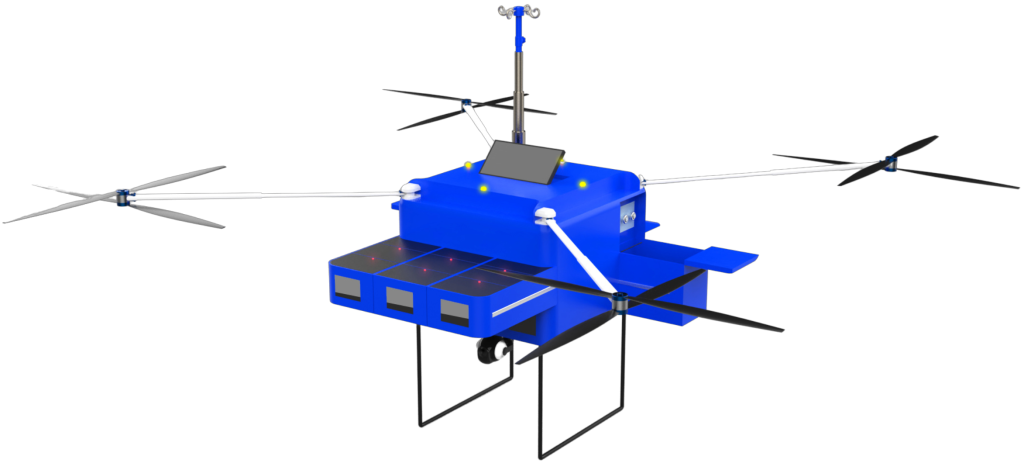
Enhanced Predictive Analysis
Enhanced predictive analysis is another significant advantage of combining AI, ML, and predictive analytics with drones. The system can further refine its predictions and guidance, leading to more accurate and effective treatments.
Personalized Care and Increased Efficiency
The utilization of AI and IoT also allows for more personalized care. The collected data can direct drones to cater to individual patient needs, making healthcare more tailored to each patient. Additionally, drones can also increase the efficiency of healthcare staff by handling transportation and preliminary monitoring tasks.
Collaboration, Training, and Psychological Comfort
By integrating with other emergency systems, drones can enable better collaboration. Moreover, they can serve as mobile platforms for training simulations, allowing medical professionals to practice in real-life scenarios. The knowledge of a rapid response possibility also provides psychological comfort to patients and their families, fostering trust in the healthcare system.
Scalability and Compliance
With the advancement of technology, the capabilities of drones can be expanded, ensuring scalability. By adhering to healthcare regulations and standards, drone-able AiMediQ MediQs can ensure a seamless and legally compliant integration into existing healthcare systems.
Disaster Response
In events of natural disasters or mass casualty incidents, drones equipped with AiMediQ technology can provide immediate response and support. This function makes them a vital tool in disaster response and large-scale emergencies.
Making the AiMediQ miniature and drone-able would not only capitalize on the existing technologies such as AI, IoT, ML, and predictive analytics but also open up new avenues to revolutionize emergency medical response, providing a more immediate, personalized, and effective care system. This innovative approach to healthcare could revolutionize emergency responses and significantly improve patient care and survival rates.



